Knowledge about the early forms of life in the universe that may have led to the development of life on Earth remains largely unknown. However, a group of scientists at the University of Hawaiʻi at Mānoa are attempting to change that.
In a newly published paper, researchers in the Department of Chemistry have discovered how some crucial molecules can form in space, which could lead to significant developments about how life may have originated on Earth.
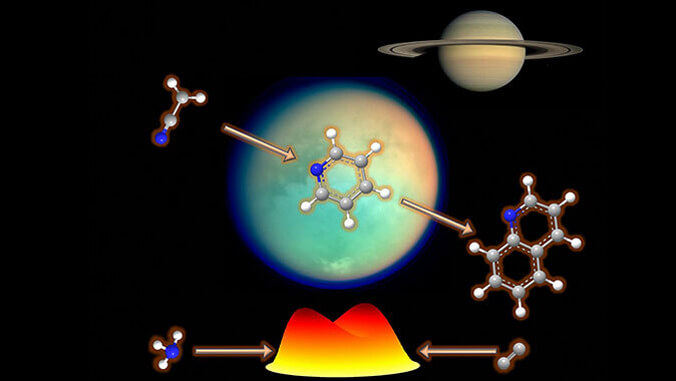
The molecules in question are called nitrogen carrying aromatic molecules, which are important in many areas of chemistry and biology. They serve as the building blocks for a wide range of compounds, including pharmaceuticals, dyes, plastics, and natural products. Aromatic molecules are also found in important biomolecules such as amino acids, nucleic acids (DNA and RNA) and vitamins.
Using molecular beams, the UH chemistry team, led by Professor Ralf I. Kaiser, recreated the environments of the Taurus Molecular Cloud (dense region of interstellar gas and dust located in the Taurus constellation, where new stars are actively forming) and of Titan’s atmosphere (resembles Earth’s early conditions due to its nitrogen-rich composition and presence of methane). Titan is Saturn’s largest moon.
In combination with electronic structure calculations by Professor Alexander M. Mebel (Florida International University), along with interstellar (Professor Xiaohu Li, Chinese Academy of Sciences) and atmospheric modeling (Professor Jean-Christophe Loison, University of Bordeaux), postdoctoral fellow Zhenghai Yang was able to pinpoint fundamental structural units of aromatic (not related to smell) molecules, which offers new paths to understanding how the building blocks of DNA and RNA might have formed in space, reshaping our ideas about how life’s ingredients originated throughout the galaxy.
“The study suggests that nitrogen carrying aromatic molecules—pyridine, pyridinyl, and (iso)quinoline—could have been synthesized in environments that scientists are really honing in on due to their similarities to Earth,” Kaiser said. “Understanding how these molecules form is vital for unraveling the mysteries of life’s origins. Findings like these could have future implications, including for practical applications not only in biotechnology and synthetic biology, but also in combustion sciences.”
See the entire paper in Nature Astronomy.
The Department of Chemistry is housed in UH Mānoa’s College of Natural Sciences.