A University of Hawaiʻi at Mānoa scientist recently discovered a protein topology that has implications for human health, earning a five-year, $670,600 grant from the National Science Foundation (NSF).
According to the National Center for Biotechnology Information, “The topology of a protein structure is a highly simplified description of its fold including only the sequence of secondary structure elements, and their relative spatial positions and approximate orientations.” The NSF CAREER award, presented to Department of Chemistry Assistant Professor Ellinor Haglund, will advance research into the Pierced Lasso Topology (PLT), which was discovered by Haglund within the past decade.
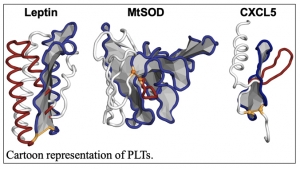
“PLTs are found in many human cell-signaling proteins, bacterial and viral proteins important in human health,” Haglund said. “Interestingly, oxidative stress, inflammation and gut microbiota may affect the chemical environment controlling the threaded topology. Thus, PLT proteins may act as molecular switches turning the activity on/off depending on the chemical environment. Understanding how biology may manipulate protein topologies and function may have a large impact on human health such as diabetes, amyolateral sclerosis (ALS), and Alzheimer’s disease, to mention a few, controlled by PLT containing proteins.”
This funded research will delve deeper into the biological role of PLT proteins. Haglund’s research team is the world’s first group focusing on PLTs. To conduct the research, they will use biochemical and biophysical methods combined with cell biology, revealing how PLT proteins thread and fold to reach their biologically active states. Haglund and graduate student Jennifer M. Simien concluded that PLTs were created in nature to control biological activity and possibly to protect against the degradation of proteins within living organisms.
This work is an example of UH Mānoa’s goal of Excellence in Research: Advancing the Research and Creative Work Enterprise (PDF), one of four goals identified in the 2015–25 Strategic Plan (PDF), updated in December 2020.