A key molecular building block in phosphorus chemistry has been elusive, until now. University of Hawaiʻi at Mānoa researchers have discovered a simple and versatile way to identify the Hückel aromatic cyclotriphosphazene molecule along with its isomer (which has the same molecular formula, but differs structurally).
According to the research team and authors—UH Mānoa Department of Chemistry Professor Ralf I. Kaiser, postdoctoral fellow Cheng Zhu, visiting researcher André K. Eckhardt, postdoctoral fellow Alexandre Bergantini, postdoctoral fellow Santosh K. Singh and Professor Peter R. Schreiner from Justus Liebig University Giessen in Germany—the study published in Science Advances represents fundamental chemistry research on chemical bonding and molecular structure. It highlights the differences and similarities between aromatic inorganic and organic compounds.
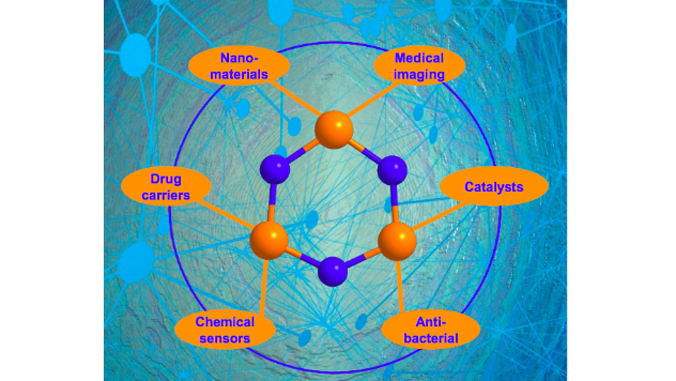
Researchers said dendrimers (repetitively branched molecules) based on the cyclotriphosphazene molecule detected in this study can be used in nanomaterials, biomolecule carriers, chemical sensors, antibacterial agents, reusable catalysts and medical imaging agents.
Key discovery
Kaiser and Zhu outlined that the cyclotriphosphazene molecule and its Dewar-benzene-type isomer were prepared within low temperature matrices of ammonia and phosphine ices exposed to ionizing radiation. Using UH Mānoa W.M. Keck Research Laboratory in Astrochemistry’s advanced photoionization, mass spectrometric devices, both species were identified, observed and analyzed. This cannot be achieved currently with traditional synthetic techniques.
“The finding helps to understand the electronic structure and chemical bonding of exotic inorganic molecules and how they relate to their classical organic counterparts,” Zhu said.
Next steps
Kaiser and Zhu said they are planning to identify more exotic isomers such as high energy density, hexaatomic prismatic molecules containing phosphorus and nitrogen. Researchers said these have been predicted computationally, but have not yet been detected experimentally.
—By Marc Arakaki