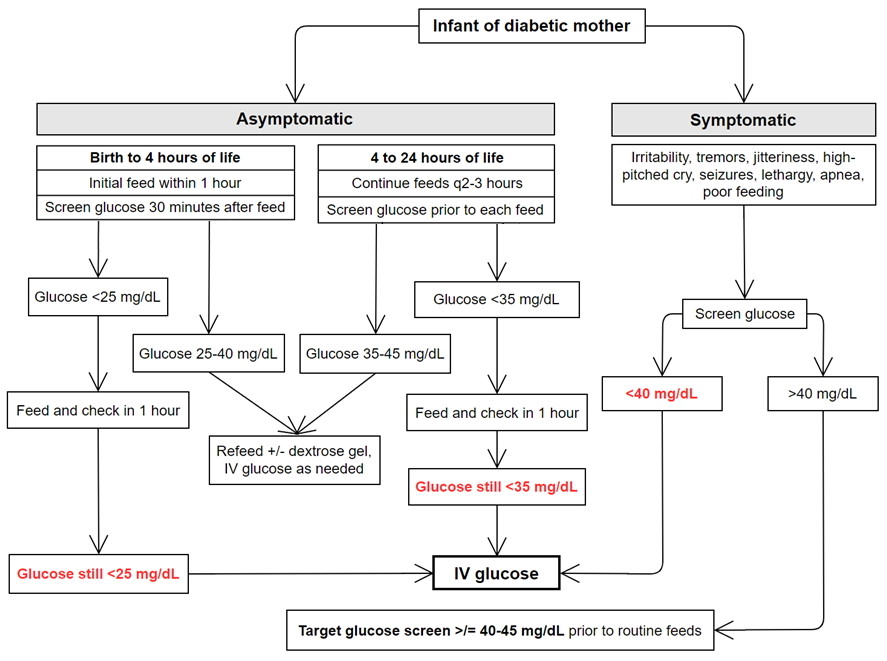
Figure 1. Screening and Management of Glucose in Infants of Diabetic Mothers, derived from AAP Clinical Report: Postnatal Glucose Homeostasis in Late-Preterm and Term Infants (5)

The editors and current authors would like to thank and acknowledge the significant contribution of the previous author of this chapter from the 2002 first edition, Dr. Joel Ruff. This current third edition chapter is a revision and update of the original authorís work.
This is a newborn infant male delivered to a 25 year old G5P3 mother at 37 weeks gestation by C-section for non-reassuring fetal heart tones. The pregnancy is notable for an antenatal ultrasound diagnosis of cleft lip and palate. Maternal serologies are unremarkable and her prenatal glucose tolerance test is normal. At delivery, continuous positive airway pressure (CPAP) is given for about 2 minutes for poor color and respiratory effort. Apgar scores are 6 (-2 color, -1 tone, -1 respiratory effort) and 9 (-1 color) at one and five minutes, respectively.
Exam: Vital signs are normal. Oxygen saturation is 99% in room air. Weight is 3950 grams, height is 54 cm and head circumference is 37.5 cm (all >95th percentile for gestational age). The infant is active and jittery with an obvious left sided cleft lip and palate. Heart is regular without murmurs. Lungs are clear. Abdomen is soft, without masses or hepatosplenomegaly. The remainder of the initial exam is normal.
The infant is transferred to the term nursery. A bedside point of care (POC) glucose is less than 30 mg/dL. A peripheral intravenous (PIV) catheter is started and 8 mL (2 mL/kg) of 10% dextrose in water (D10W) is given IV. The blood glucose from the lab that was drawn prior to the dextrose bolus results as 20 mg/dL. The infant is transferred to the intermediate nursery where a repeat POC glucose 30 minutes after the dextrose bolus is still <30 mg/dL. A second IV bolus of D10W is given. IV D10 is running at 80 mL/kg/day, which gives a glucose infusion rate of 5.6 mg/kg/minute. A blood glucose obtained 30 minutes after a second dextrose bolus is 31 mg/dL. The fluid is changed to 12.5% dextrose (D12.5W), the maximum dextrose concentration that can run in a PIV, at the same IV rate. A third POC glucose checked 30 minutes after the IV fluid change is still below 40 mg/dL and so a third dextrose bolus is given and the D12.5W infusion is increased to 100 mL/kg/day, which is a glucose infusion rate of almost 9 mg/kg/minute, and the baby is offered some formula. The next glucose check 30 minutes later is 48 mg/dL. The infant requires 100 mL/kg/day of dextrose 12.5% for about 24 hours before blood glucose levels stabilize and weaning of the IV rate is attempted.
Neonatal hypoglycemia is a very common condition and can be transient in healthy neonates immediately after birth. It is considered transitional hypoglycemia if the blood glucose levels improve and reach normal ranges within 24 to 48 hours after birth with an established feeding cycle. 10% of healthy term newborns present with transitional hypoglycemia (1). If the hypoglycemia is more prolonged and severe, which occurs in a minority of neonates, there is a concern for persistent hypoglycemia and associated neurologic morbidity (2,3). The incidence of hypoglycemia is significantly higher in certain subgroups: 8% in large for gestational age (LGA) infants and about 15% in small for gestational age (SGA) infants (1). Neonatal hypoglycemia can be easily treated in most cases if it is recognized, but untreated hypoglycemia can have serious consequences for the infant since glucose is the major substrate for energy in all organs and almost exclusively used for cerebral metabolism (1,4).
The terms glucose and dextrose are used almost interchangeably. Technically, dextrose is the D stereoisomer of glucose (i.e., D-glucose). D10W is 10% dextrose (10 grams dextrose per 100 grams of water or 10,000 mg dextrose per dL), thus, when IV fluids are being described, the term dextrose is used. A rapid infusion of D10W is a dextrose bolus, but it is also very common to call this a glucose bolus. When blood, plasma, or serum glucose is measured, we almost never refer to this as blood, plasma, or serum dextrose. This chapter uses the terms glucose and dextrose almost interchangeably.
Glucose is the metabolic fuel for the developing fetus and its brain. Serum glucose levels are about 70% of those in the mother (i.e., baby's glucose is about 70 mg/dL, when mother's glucose is 100 mg/dL) and almost all of this comes from facilitated diffusion across the placenta. The fetus produces its own insulin to maintain euglycemia. Stores of glycogen in the liver accumulate slowly through gestation with a marked increase during the last trimester.
After delivery, the maternal supply of glucose is interrupted. Glycogen phosphorylase breaks down hepatic glycogen stores to supply glucose to the newborn. Gluconeogenesis accounts for approximately 10% of the glucose source in newborns by several hours of age (1,5-6). A term infant is estimated to have only enough hepatic glycogen to support metabolic demands for about 10 hours without an exogenous energy source. At the same time, synthesis of enzymes involved in gluconeogenesis increases and catecholamine levels are high (stimulating the release of substrates in the form of free fatty acids and free amino acids). Blood glucose levels in all infants take a physiologic dip in the first 2-3 hours of life and then spontaneously increase to a stable level with regular feedings (1). Some studies show that the drop in blood glucose soon after birth may be important in stimulating the necessary physiological processes to maintain blood glucose (i.e., gluconeogenesis, glycogenolysis, oxidative fat metabolism, appetite stimulation) so that the infant can survive in the extrauterine environment (6). Neonates can initially compensate for the physiologic hypoglycemia by producing ketones bodies generated from fatty acid oxidation. If there is an imbalance between the supply and demand of glucose and alternative fuels secondary to a variety of dysregulated mechanisms, clinically significant neonatal hypoglycemia can occur (4). Neonates must have a consistent and frequent feeding schedule during the first few days after birth to meet the bodyís continued demand for glucose. Higher insulin levels after birth in infants who are at risk for hypoglycemia can augment the drop in blood glucose during the 1st few hours of life (3). Some neonates are unable to maintain the standard blood glucose concentrations due to limited food availability, poor oral intake, or impaired metabolism resulting in severe and persistent hypoglycemia and subsequently neurologic morbidity, including seizures (1).
Hypoglycemic newborn infants may present with a variety of symptoms or be entirely asymptomatic. Signs and symptoms can be categorized as neurogenic or neuroglycopenic. Neurogenic symptoms occur from the activation of the sympathetic nervous system in response to hypoglycemia. Patients with neurogenic symptoms will appear diaphoretic, pale, irritable, hungry, tachycardic with tremors, exhibit temperature instability, and have vomiting. Neuroglycopenic symptoms occur from the deprivation of glucose from the central nervous system. Patients with neuroglycopenic symptoms can present with seizures or apnea if hypoglycemia is not treated (1).
A convenient way to consider the differential is to divide it into transient hypoglycemia or persistent/recurrent hypoglycemia. The latter is defined as requiring IV dextrose beyond 48 hours after birth (7). Distinguishing between the two is critical for early diagnosis and management and prevention of serious neurological consequences (seizures and permanent brain injury) (8).
Table 1. Transient and persistent causes of neonatal hypoglycemia (1,3-4,6)
| Transient hypoglycemia | Persistent/recurrent hypoglycemia |
| Maternal conditions: -Infant of diabetic mother (3-4,6) or other infants who were exposed to elevated maternal glucose which often leads to LGA size -Use of beta-blockers (3) | Maternal conditions: -Maternal pre-eclampsia/eclampsia or hypertension (6) -Family history of hypoglycemia (6) |
| Neonatal conditions: -Inadequate hepatic glycogen stores (1,4) -Inadequate muscle stores as a source of amino acids (1,4) -Inadequate lipid stores as a source of fatty acids (1,4) -Prematurity (3) -Sepsis (3) -Perinatal Stress | Neonatal conditions: Hyperinsulinism -Congenital hyperinsulinemia, previously known as primary islet cell hypertrophy or nesidioblastosis (1,3) -Beckwith-Wiedemann syndrome (3, 5-6) Low Glycogen Stores -SGA resulting in low glycogen stores (1,4) Perinatal stress -Birth asphyxia/ischemia (3) -Cesarean delivery for fetal distress (6) -Intrauterine growth retardation (IUGR) (1,6) -Meconium aspiration syndrome, erythroblastosis fetalis, polycythemia (3) -Hypothermia (6) Endocrine deficiency -Pituitary insufficiency: hypopituitarism, growth hormone deficiency, cortisol deficiency (1,3) -Congenital glucagon deficiency Inborn errors of metabolism (1,3-4): -Carbohydrate metabolism: Galactosemia, glycogen storage disease, fructose intolerance -Amino acid metabolism: Maple syrup urine disease, propionic acidemia, methylmalonic aciduria, tyrosinemia, glutaric acidemia -Fatty acid metabolism: Carnitine metabolism defect, Acyl-CoA dehydrogenase defect |
Table 2. Causes of neonatal hypoglycemia categorized by physiological mechanism (1)
| Physiologic Mechanism | Associated Disorder |
| Inadequate glycogen stores and substrate source for gluconeogenesis | -Prematurity -Small for gestational age -Intrauterine growth restriction -Perinatal stress (sepsis, asphyxia) -Polycythemia |
| Hyperinsulinism | -Infant of diabetic mother -Beckwith-Wiedemann syndrome -Congenital hyperinsulinism -Soto syndrome |
| Growth hormone deficiency | -Turner mosaicism -Costello syndrome -Hypopituitarism |
| Cortisol deficiency | -Costello syndrome -Hypopituitarism -Congenital adrenal hyperplasia |
| Amino acid abnormalities | -Maple syrup urine disease |
| Glycogen | -Hepatic glycogen storage diseases |
| Glucose | -Hereditary fructose intolerance -Galactosemia |
| Fatty acids | -Medium and short-chain acyl-coenzyme A dehydrogenase deficiency -Carnitine palmitoyltransferase deficiency types I and II |
Your first evaluation of hypoglycemic infants should be of airway, breathing and circulation (ABCs) and performing adequate cardiorespiratory resuscitation or support if needed. The difference in accuracy in blood glucose (BG) values can depend on the source of the sample, whether it is from plasma or whole blood. Laboratory plasma glucose values can be approximately 10% to 18% greater than whole blood point of care (POC) glucose measurements (1,5). Variation of POC blood glucose measurements when compared to plasma glucose laboratory measurements can range from 10 to 20 mg/dL. At the extremes of glucose values (very low or very high), the POC glucose measurements tend to be less accurate, which is why laboratory confirmation of a low POC blood glucose value is needed, especially if the patient is symptomatic.
Initial evaluation should evaluate the motherís current milk supply and the infantís ability to feed as well as an assessment for history of risk factors such as peripartum stress, maternal risk factors, perinatal asphyxia, and infection. For infants with persistent hypoglycemia, additional laboratory workup is needed and may include checking insulin levels to assess for hyperinsulinism or cortisol and growth hormones to assess for hypopituitarism or adrenal insufficiency (1). Depending on the results of the initial laboratory evaluation, additional labs to consider would be lactic acid, ammonia, urinary ketones, beta-hydroxybutyrate, free fatty acids, acylcarnitine profile, plasma amino acids, and urine organic acids. Further consultation with endocrinology, genetics, or metabolic specialists may also be warranted.
Asymptomatic infants at high risk of hypoglycemia should be screened soon after birth (1). These include: 1) SGA (below statistical 10th percentile for gestational age), 2) LGA (above statistical 90th percentile for gestational age), 3) infant of diabetic mothers (IDM), 4) preterm infants (<37 weeks). Regardless of risk factors, all infants with symptoms possibly related to hypoglycemia should be screened immediately with a glucose check.
There is controversy on the definition of neonatal hypoglycemia, since the range over which symptoms of hypoglycemia appear can vary widely (4). The definition of hypoglycemia may vary according to hours of life and the presence of clinical symptoms from patient to patient. All infants experience a physiological low point in their blood glucose in the first 2 to 3 hours of life, which can reach as low as 30 mg/dL in healthy asymptomatic newborns (1). A healthy term infant in no distress may tolerate 30 mg/dL well but a stressed, premature infant may be symptomatic at 50 mg/dL. Many infants with low blood sugars can have no risk factors and be clinically asymptomatic while others can have poor oral intake or experience longer intervals between substantial feedings but can be clinically asymptomatic. The generally accepted definition for neonatal hypoglycemia ranges from glucose levels below 36 to 47 mg/dL.
The guidelines for management of neonatal hypoglycemia differ depending on the organization putting forth the guidelines. The American Association of Pediatrics (AAP) focuses on identifying transitional hypoglycemia within the first 24 hours after birth and offers guidance on screening 1) late and preterm infants who are symptomatic and 2) asymptomatic infants who are at risk (1,4). Frequency and duration of screening are related to infantís risk factors. IDMs should be screened as early as 1 hour of age. LGA or SGA can present with low glucose levels by 3 hours of life and are at risk for neonatal hypoglycemia for up to 10 days after birth (4).
The Pediatric Endocrine Society focuses on identifying infants at risk for persistent hypoglycemia at >48 hours after birth, excluding infants with transient hypoglycemia (9). In particular, there is a lower glucose goal for high-risk neonates without suspected congenital hypoglycemia (50 mg/dL for <48 hours after birth; 60 mg/dL for >48 hours after birth) in comparison to infants with suspected congenital hypoglycemia (70 mg/dL). The rationale in having a lower threshold for the former group is to minimize the risk of intervention (i.e. separation of mother and baby, reducing the opportunities for breastfeeding) at the expense of potential undertreatment (1,9). Furthermore, the treatment target for infants with suspected genetic hypoglycemia and symptomatic infants is higher to reduce the risk of undertreatment and to prevent neurological injury in severe, persistent hypoglycemia (1,9).
The goal of treatment is to establish normoglycemia, usually defined as a stable glucose value above 40 mg/dL in the first 48 hours of life and above 60 mg/dL after 48 hours of life before each feeding. Regular feedings can be scheduled for every 2 to 3 hours with glucose measurements obtained just prior to feeding. High-risk infants should be fed via breastfeeding or formula within the first hour of life and screened for hypoglycemia within 30 minutes after feeding. Gavage (tube) feeding can be offered for infants with poor oral function.
The duration of glucose screening is dependent on the patientís risk factors and clinical presentation. If the plasma glucose level remains below 45 mg/dL, glucose screening before feeding should continue for the next 24 hours (4). IDMs and LGA infants who can maintain a glucose above 40 mg/dL will undergo routine glucose screening until 12 hours of age. Serial glucose measurements for late preterm and SGA infants may continue until at least 24 hours after birth (5).
The goal is to keep the serum glucose levels between above 40 mg/dL in the first two days of life for stable asymptomatic infants (5). In general, all symptomatic infants with a glucose level <40 mg/dL require IV glucose therapy. At-risk asymptomatic infants with glucose levels <25 mg/dL within the first 4 hours of life or <35 mg/dL within 4 to 24 hours of life, should be refed and have glucose levels measured within 1 hour after feeding. If glucose levels remain <35 mg/dL despite feeding or if the infant is unable to tolerate feeds, IV glucose therapy should be considered (5). Obtain a laboratory plasma sample before starting treatment. IV glucose therapy includes a bolus of 200 mg/kg of dextrose, which is usually administered as 2 mL/kg bolus of D10W followed by an infusion of continuous glucose with a glucose infusion rate (GIR) of 6 to 8 mg/kg/min, which is approximately 80 to 120 mL/kg/day of D10W (5)
The formula to calculate the GIR (in mg/kg/min) is [(IV rate mL/hr) x (Dextrose %)] / [(weight in kg) x 6].

Figure 1. Screening and Management of Glucose in Infants of Diabetic Mothers, derived from AAP Clinical Report: Postnatal Glucose Homeostasis in Late-Preterm and Term Infants (5)
An alternative to IV glucose therapy can be a 40% dextrose gel that is administered orally. A research study published in 2015 by Harris et al showed that this treatment is more effective than feeding alone in reversing neonatal hypoglycemia in at-risk late preterm and term infants in the first 48 hours of life. Advantages to dextrose gel include low cost, ease of administration, allowing mother and baby to stay together and more opportunities for breastfeeding by avoiding neonatal intensive care unit (NICU) admission (10). Dextrose gel has become the first line treatment to manage hypoglycemia in late preterm and term babies in the first 48 hours of life.
For infants who remain hypoglycemic after initiation of IV fluids, the GIR will need to be increased by either increasing the IV rate or increasing the dextrose concentration. However because of higher osmolarity, peripheral veins cannot tolerate more than 12.5% dextrose. An infant requiring a higher glucose concentration will require a central venous line. Some infants may require glucose infusion rates as high as 16 to 20 mg/kg/min, but any infant in this range needs further evaluation and consultation with an endocrinologist.
Other pharmacological interventions that may be considered for persistent hypoglycemia include hydrocortisone (after checking a baseline cortisol level) to enhance gluconeogenesis in the liver and reduce insulin sensitivity; intramuscular glucagon to stimulate the conversion of glycogen to glucose (11); diazoxide and octreotide to decrease pancreatic insulin secretion in the setting of hyperinsulinism (12); and nifedipine to reduce glucose tolerance and insulin secretion (1). Long term treatment for those with inborn errors of metabolism such as glycogen storage diseases should be managed by a multidisciplinary team led by a physician with experience in this field, with the involvement of a dietitian, social worker, geneticist, and other specialists as needed (endocrinologist, nephrologist, hepatologist, etc) (13).
The intravenous glucose infusion may be weaned after the glucose has been stable and in the normal range for at least 12 to 24 hours (1) or even longer, especially for babies who have had difficulty in achieving euglycemia. According to the AAP, it is important to ensure that the infant can maintain normal plasma glucose levels through at least 3 feeding-fasting cycles before they can be discharged from the hospital (5). If the infant is unable to reach the target glucose goal after 24 hours of glucose infusion, an evaluation and work up for hyperinsulinemic hypoglycemia is warranted. Hyperinsulinemia hypoglycemia is the most common cause of severe persistent neonatal hypoglycemia (5).
Although there is limited evidence that prolonged hypoglycemia can cause severe neurological effects, empirical screening recommendations have been established to optimize the identification and treatment of neonatal hypoglycemia (1). In particular, neonates with low glucose availability and inefficient ketone body production due to inborn errors of metabolism or other congenital disorders causing hypoglycemia, who experience severe and prolonged hypoglycemia may be at greatest risk for long term effects. The most concerning outcome of neonatal hypoglycemia is seizures that can progress to hypoxic brain injury, leading to severe adverse neurodevelopmental outcomes or death. Severe and prolonged neonatal hypoglycemia is associated with cerebral damage, including negative effects to the dentate gyrus, subiculum, hippocampus, and caudate-putamen nuclei (14). The mechanism for neuronal damage involves neuronal depolarization induced by hypoglycemia, elevated release of the neurotransmitters glutamate and aspartate, leading to neuronal excitotoxicity, and ultimately neuronal death (14). The occipital lobe injury has also been reported with recent studies showing increased risk of visual-motor impairment and executive dysfunction at 2 to 5 years of life (2), however it is not associated with neurodevelopmental impairment during the same years of life. There is also a greater risk of low language and literacy skills for children between the ages of 6 to 11 years old (2). The risk of neurodevelopmental impairment secondary to hypoglycemia is greater in infants who are preterm, SGA, IDM, which may be due to the abnormal intrauterine environment or shortened gestation (6,10).
The complications from neonatal hypoglycemia can be prevented by promptly identifying at-risk neonates, initiating early feeding, and having robust support for breastfeeding mothers. Urgent evaluation and initiation of treatment when an infant presents with signs of hypoglycemia can be beneficial in preventing central nervous system effects (1).
Questions
1. True/False: The level of hypoglycemia resulting in serious sequelae is well defined by scientific studies.
2. When evaluating a hypoglycemic infant, the first thing to assess is:
a. Ballard exam
b. Presence of red reflex
c. Airway, breathing, circulation
d. Presence or absence of a suck reflex
3. What is the formula to calculate the glucose infusion rate and at what level should you start?
4. Which of the following infants are at risk for hypoglycemia and should have a screening blood glucose performed in the nursery?
a. Infant of diabetic mother
b. A jittery infant
c. Small for gestational age infant status post difficult delivery
d. 37 week infant born to a GBS positive mother but has no ill symptoms
References
1. Thompson-Branch A, Havranek T. Neonatal Hypoglycemia. Pediatr Rev. 2017;38(4):147-157. doi:10.1542/pir.2016-0063
2. Paulsen ME, Rao RB. Cerebral Effects of Neonatal Dysglycemia. Clin Perinatol. 2022;49(2):405-426. doi: 10.1016/j.clp.2022.02.008. Epub 2022 Apr 21. PMID: 35659094; PMCID: PMC9177056.
3. Alsaleem M, Saadeh L, Kamat D. Neonatal Hypoglycemia: A Review. Clin Pediatr (Phila). 2019;58(13):1381-1386. doi:10.1177/0009922819875540
4. Adamkin DH. Neonatal hypoglycemia. Semin Fetal Neonatal Med. 2017;22(1):36-41. doi:10.1016/j.siny.2016.08.007
5. Adamkin D and Committee on Fetus and Newborn. Postnatal glucose homeostasis in late-preterm and term infants. Pediatrics 2011;127(3):575-679.
6. Garg M, Devaskar SU. Chapter 86. Disorders of Carbohydrate Metabolism. In: Fanaroff AA, Martin RJ (eds). Neonatal-Perinatal Medicine, 11th edition, 2020. Elsevier, Philadelphia, PA, pp. 1584-1610.
7. Stark J, Simma B, Blassnig-Ezeh A. Incidence of hypoglycemia in newborn infants identified as at risk. J Matern Fetal Neonatal Med. 2020;33(18):3091-3096. doi:10.1080/14767058.2019.1568985
8. Makker K, Alissa R, Dudek C, Travers L, et al. Glucose Gel in Infants at Risk for Transitional Neonatal Hypoglycemia. Am J Perinatol. 2018;35(11):1050-1056. doi:10.1055/s-0038-1639338
9. Thornton PS, Stanley CA, De Leon DD, et al. Recommendations from the Pediatric Endocrine Society for Evaluation and Management of Persistent Hypoglycemia in Neonates, Infants, and Children. The Journal of pediatrics. 2015;167(2):238-245. doi:10.1016/j.jpeds.2015.03.057
10. Harris DL, Alsweiler JM, Ansell JM, et al. Outcome at 2 Years after Dextrose Gel Treatment for Neonatal Hypoglycemia: Follow-Up of a Randomized Trial. J Pediatr. 2015;170:54-59.e2. doi:10.1016/j.jpeds.2015.10.066
11. Kasirer Y, Dotan O, Mimouni FB, Wasserteil N, et al. The use of intramuscular glucagon to prevent IV glucose infusion in early neonatal hypoglycemia. J Perinatol. 2021;41(5):1158-1165. doi:10.1038/s41372-021-00925-x
12. Brar PC, Heksch R, Cossen K, et al. Management and Appropriate Use of Diazoxide in Infants and Children with Hyperinsulinism. J Clin Endocrinol Metab. 2020;105(12):dgaa543. doi:10.1210/clinem/dgaa543
13. Kishnani PS, Austin SL, Abdenur JE, Arn P, et al, American College of Medical Genetics and Genomics. Diagnosis and management of glycogen storage disease type I: a practice guideline of the American College of Medical Genetics and Genomics. Genet Med. 2014 Nov;16(11):e1. doi: 10.1038/gim.2014.128. PMID: 25356975.
14. De Angelis LC, Brigati G, Polleri G, et al. Neonatal Hypoglycemia and Brain Vulnerability. Front Endocrinol (Lausanne). 2021;12:634305. doi:10.3389/fendo.2021.634305
Answers to questions
1. False
2. c
3. GIR (in mg/kg/minute) = [(IV rate mL/hr) x (Dextrose %)] / [(weight in kg) x 6]. Start at 6 to 8 mg/kg/min and titrate.
4. a, b and c are all correct.