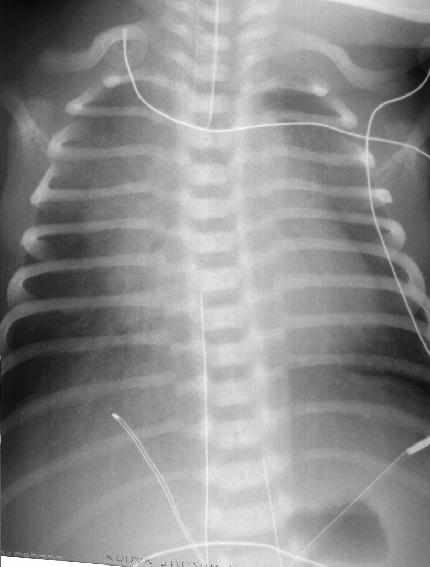
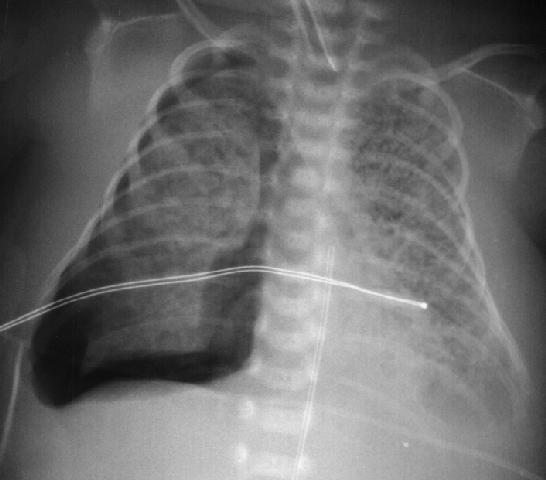
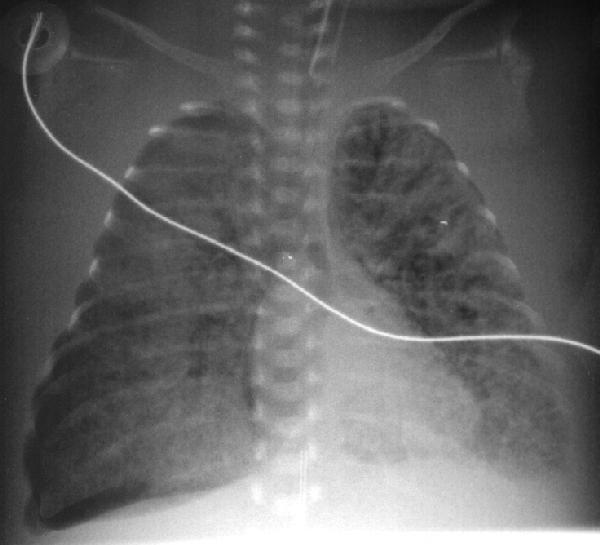
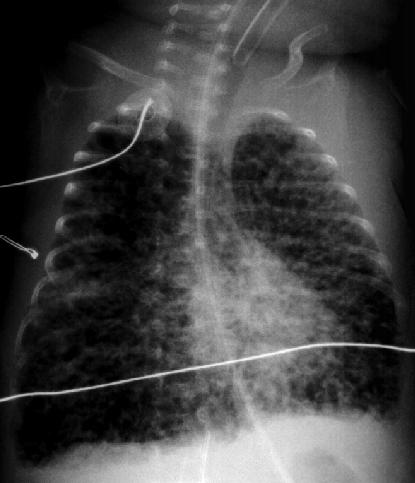
A 2 hour old, 38 week gestation, 3 kg male infant was born to a 25 year old G1P1 GBS unscreened woman with otherwise unremarkable prenatal labs who had an uncomplicated pregnancy, labor and delivery. Apgar scores were 9 and 9. He was sent to the newborn nursery. He breast fed 1 hour ago without concerns. He now presents with respiratory distress.
Exam: VS: T37, HR 160, RR 80, BP 60/35 (mean 45 mmHg), Osat 95% on 2 L/min of heated humidified 25% FiO2 by nasal cannula. Weight, height, and head circumference are at the 50th percentile. He is a term male with obvious respiratory distress and tachypnea. His chest shape is symmetric with equal and clear breath sounds. Mild to moderate chest retractions are present but breath sounds are otherwise clear with good aeration and equal bilaterally. Heart is regular with a normal S1, split S2, and no murmurs. His pulses are normal. The remainder of his physical exam is normal.
Laboratory results: CBC: WBC 15,000, 8% bands, 50% neutrophils, 40% lymphocytes, 2% monocytes, hemoglobin18.5 gm/dL, hematocrit 55%, platelets 250,000. No toxic granulations or vacuoles of the neutrophils are noted. ABG: pH 7.35, pCO2 55 mmHg, pO2 70 mmHg, BE -7 on 2L/min oxygen via nasal cannula. Chest x-ray: 10 ribs of inflation, streaky linear perihilar densities, and small scattered patchy densities bilaterally.
Over the next several hours, the infant develops progressively more distress and a greater oxygen requirement. He is now requiring 50% FiO2.
What is your differential diagnoses? How would you manage this infant?
Respiratory distress is one of the most common presenting problems of newborns. The constellation of signs and symptoms can be the result of pulmonary, cardiac, metabolic, infectious, renal, gastroenterologic, and neurologic pathologic processes. Newborns with disorders involving any one of these organ systems may present with varying degrees of respiratory distress including symptoms such as tachypnea, retractions, or grunting.
Transient Tachypnea of the Newborn
Transient tachypnea of the newborn (TTN) is the most common respiratory disorder of the term or near term newborn. It results from newborn’s inability to clear fluid from their lungs following birth. Approximately 100 mL of fluid needs to be cleared from a newborn’s lungs to initiate extrauterine breathing. Fetal stress during labor and birth induces an increased release of fetal epinephrine. Epinephrine activates the chloride and sodium channel pumps in the fetal airways causing the absorption, rather than secretion, of fetal lung fluid. The mechanical squeezing of the newborn’s chest during labor and vaginal delivery is also responsible for a small portion of lung fluid expulsion. In a healthy newborn, the majority of the lung fluid is cleared within 2 to 6 hours after birth (1). Failure of the above process, or failure to clear the lungs due to increased fluid, leads to TTN. TTN is also associated with Cesarean delivery, particularly when done prior to the onset of labor. TTN is a diagnosis of exclusion with tachypnea as the primary clinical feature. Typically, the infant becomes tachypneic shortly after birth and exhibits varying degrees of grunting, nasal flaring, and retractions. Some patient might demonstrate a barrel-shaped chest because of lung hyperinflation (2). Chest radiographs (CXR) reveal hyperinflation with retained fluid demonstrated by diffuse streaky pulmonary interstitial infiltrates, interlobar septae edema, and fluid in the fissures. There should be no areas of consolidation. TTN is self-limited with most cases resolving within 48 hours. Radiographs should show improvement by 48 to 72 hours with complete resolution within 7 days. A worsening clinical picture should suggest another diagnosis.
Meconium Aspiration
Meconium in the amniotic fluid, also known as meconium-stained amniotic fluid (MSAF), is often a sign of in-utero fetal distress and occurs in approximately 20% of all pregnancies. Meconium aspiration syndrome (MAS) occurs in 3% to 12% of infants with MSAF. MAS may only occur in the presence of MSAF. The incidence of MAS increases with gestational age between 38 and 42 weeks (3). Term and post term infants have more meconium and are more likely to pass meconium into the amniotic fluid prior to delivery. Other substances such as blood or amniotic fluid can also be aspirated by the newborn infant. Since meconium aspiration is a common problem, much effort has been made over the last 30 years to prevent this condition by reducing intrapartum and postpartum aspiration.
The pathophysiologic mechanism is multifaceted and not well understood. The partial or total obstruction of small airways with the aspirated material (meconium, blood, amniotic fluid contents) may lead to hyperinflation and/or atelectasis. It is believed that meconium, particularly bile salts, inactivates surfactant. Poor transition to extrauterine life may also result in impaired pulmonary vasculature relaxation. The plugging of the airways and resultant inflammation of the lung parenchyma may lead to pulmonary hypertension, further increasing oxygen and ventilation requirements of the fetus (3). As pulmonary hypertension worsens, right to left shunting can occur through the ductus arteriosus or the foramen ovale resulting in hypoxia in a condition known as persistent pulmonary hypertension (PPH), which is the most severe form of MAS. It many ways, the abnormal right to left blood flow caused by pulmonary hypertension is similar to cyanotic congenital heart disease (right to left shunting).
Like TTN, meconium aspiration syndrome is a clinical diagnosis. While many infants have the onset of symptoms at birth, some infants have an asymptomatic period of several hours before pulmonary hypertension worsens and respiratory distress becomes apparent. As the disease progresses, the symptoms and severity of hypoxemia increase over minutes to hours. Severity progression should alert the clinician to the diagnosis of aspiration syndrome and/or pulmonary hypertension, rather than TTN. The CXR may show significant heterogeneous parenchymal disease with hyperinflated and hypo-inflated areas, patchy and linear infiltrates, and/or atelectasis (1).
Mechanical ventilation is indicated in infants with severe hypoxia or respiratory acidosis. Infants requiring intubation may also receive inhaled nitric oxide (iNO) which relaxes blood vessels in the lungs, reducing pulmonary hypertension, increasing lung perfusion, and improving oxygenation (3). Clinicians have begun administering surfactant in severe cases because meconium aspiration might inactivate endogenous surfactant, which leads to reduction in duration of mechanical ventilation, the need for extracorporeal membrane oxygenation therapy (ECMO), and length of hospital stay (4). ECMO may be used in severe cases refractory to the above treatments. Although previously indicated, the American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Pediatrics (AAP) no longer recommend suctioning of the oropharynx at the perineum in all newborns with meconium in the amniotic fluid since it was not shown to improve outcomes (5,6).
Respiratory Distress Syndrome
Respiratory distress syndrome (RDS) is the most common respiratory disorder of the premature infant. Most infants are premature, less than 34 weeks gestation. The incidence and severity increase with decreasing gestation age.
While the fetal lung's structural immaturity and fluid content contributes to the pulmonary dysfunction, the major reason for this disorder is surfactant deficiency. Without surfactant, the surface tension of the alveolar sacs is high, leading to an increased tendency of the alveoli to collapse (7). As with TTN, the premature infant's lungs may not have adequate clearance of the pulmonary lung fluid. Untreated, the severity of respiratory distress progresses over time. In the first few hours after birth, the infant’s oxygen requirements will progressively increase.
Classic findings include cyanosis, grunting, nasal flaring, intercostal and subcostal retractions, and tachypnea. Grunting is the infant's attempt to maintain the pressures and gas volume within the lung by keeping the glottis is partially closed during exhalation to maintain alveolar distending pressure during exhalation. The CXR reveals hypo-inflation bilaterally with diffuse symmetrical reticulogranular (ground glass or frosted glass appearance) lung fields and air bronchograms. The frosted glass pattern is due to juxtaposed air-filled alveoli and atelectatic alveoli. Air bronchograms are visible because air-filled larger airways overly fluid filled lung tissue.
The treatment of RDS generally includes positive pressure ventilation and artificial surfactant replacement. The phospholipids and surfactant related proteins contained in surfactant spread along the air liquid interface to reduce alveolar surface tension, requiring less pressure to maintain alveolar inflation (8). Several types of animal-derived and synthetic surfactants have been approved for clinical use. Both types have demonstrated equivalent decreases in morbidity and mortality of infants with RDS (9).
Classically, surfactant suspension is administered into the lungs through a catheter or endotracheal (ET) tube. The infant’s respiration is supported via continuous positive airway pressure (CPAP) or mechanical ventilation (9). Moderately premature infants (29 to 34 weeks gestation) are usually extubated within several days after treatment. However, extremely premature infants (23 to 28 weeks gestation) may continue to require positive pressure respiratory support for several weeks.
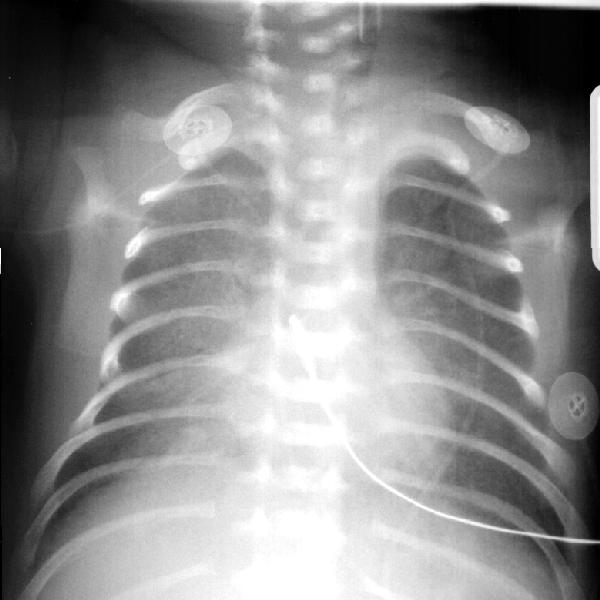
Figure 1 – RDS. Ground glass/frosted glass appearance of the lungs. Some air bronchograms are visible in the right lung and over the heart. The endotracheal tube (ETT) is too high. A temperature probe wire is visible in the center of the chest. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Figure 2 – RDS with ground glass/frosted glass appearance of the lungs. Air bronchograms are visible over the heart. The end of the ETT is just above the carina. There are two umbillical lines visible. Line on the patient’s left is the umbillical artery catheter at about T6-T7. The line on the right is an umbillical vein catheter that is too high. Other lines see are EKG wires and a temperature probe wire. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Air Leak Syndrome
The sudden onset of significant respiratory distress in a newborn should raise the possibility of an air leak syndrome. The most common air leak syndromes are pneumomediastinum and pneumothorax. Pneumopericardium is another air leak entity but it is very uncommon. In addition to respiratory distress, a severe air leak condition may cause hypotension (due to decreases in cardiac output), muffled heart tones, abdominal distention, asymmetric chest shape, and deviation of the cardiac sounds.
If time permits, air leak syndromes can be diagnosed via CXR. Free air in the hemithorax and a visible edge of the collapsed lung indicate a pneumothorax. In a pneumomediastinum, vertical air densities can be visible in the neck, mediastinum, and along the path of the trachea, elevation of the thymus with a sail or bat wing sign can be present. In a pneumopericardium, the heart will be outlined with a halo of air (10).
A pneumothorax that occurs while on positive pressure ventilation is sometimes a tension pneumothorax which means that the air in the hemithorax is under pressure which compresses the ipsilateral lung, but it also compresses the heart and the contralateral lung resulting in sudden and severe hypoxia, hypotension, and bradycardia. See figure 3. A tension pneumothorax with sudden and severe symptoms must be treatment immediately without a CXR via needle decompression (thoracentesis) and tube thoracostomy (chest tube). In tiny infants, it can be difficult to tell which side the pneumothorax is on. Auscultation might not be helpful since both lungs are compressed. A transilluminator can be used; if placed in the center of the chest, the affected side might glow brighter than the other side. If the side cannot be determined, needle decompression of one side must be chosen and if a rush of air is confirmed, this should be followed by tube thoracostomy. If a rush of air is not confirmed, then needle decompression should be attempted on the other side and if still not successful, a pneumopericardium should be suspected and a pericardiocentesis should be attempted. If a CXR is obtained in a tension pneumothorax during this resuscitation process, the mediastinum will be deviated to the opposite (contralateral) side and the ipsilateral diaphragm will be depressed.

Figure 3 – In a tension pneumothorax, air enters the pleural space during ventilator inspiration. During exhalation, the air leak forms a valve trapping the air in the pleural space. During the next inspiration breath, the ventilator pumps more air into the pleural space making the affected lung smaller and the pneumothorax larger; but more importantly is compresses the heart so that it cannot fill and pump and it compresses the contralateral lung. Oxygenation declines precipitously associated with bradycardia and hypotension necessitating immediate needle decompression and tube thoracostomy. Copyright Loren Yamamoto, 2023.
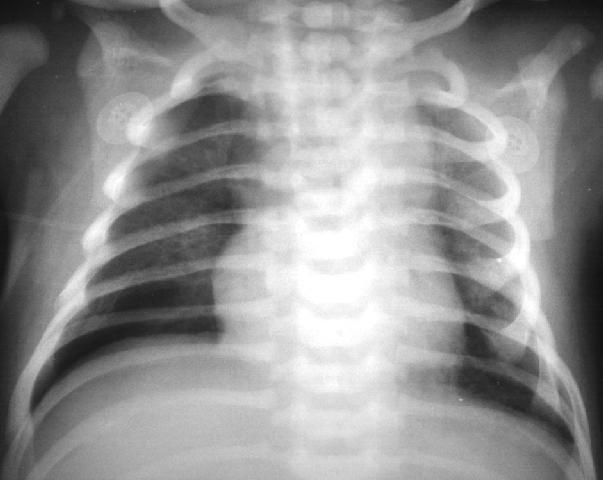
Figure 4 – The lungs are hazy, there are lucencies in the right hemithorax. Subtle right pneumothorax. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Figure 5 – Right pneumothorax, pulmonary interstitial emphysema (PIE). Note the appearance of the lungs with tiny pockets of air dissecting within the lung parenchyma. The endotracheal tube is too high at the level of the clavicles. The tip of the unbillical arter cathether is at T8. The horizontal line is a temperature probe wire. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Air leaks associated with minor symptoms may be followed clinically for spontaneously resolution. Pneumomediastinum does not require drainage. In cases other than a bronchopleural fistula, the air leak will usually seal within a few days.
Pulmonary interstitial emphysema (PIE) occurs when tiny amounts of air dissect within the lung parenchyma leading to a CXR that shows lungs with tiny patches of air alternating with lung parenchyma. This can eventually lead to a pneumothorax. See figures 5, 6, and 7.
Figure 6 – Pulmonary intersticial emphysema (PIE). There might be a small pneumothorax in the right lower hemithorax. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Figure 7 – Severe pulmonary intersticial emphysema (PIE). The lungs are hyperinflated which is why they appear blacker. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
Pneumonia and Sepsis
Infectious pneumonia in newborns largely affects preterm infants compared to their term counterparts. Mothers with intrapartum fever and prolonged rupture of membranes (greater than 18 hours) have a greater risk of transmitting infections to their infants. This risk can be reduced by administering intrapartum antibiotics for mothers with fever, prolonged rupture of membranes, or for those who are group B Streptococcus (GBS) carriers. However, the use of ampicillin for the GBS infections has increased the incidence of ampicillin resistant coliform infections. The most common bacterial organisms that infect neonates are GBS, E. coli, and Listeria monocytogenes.
Infected infants present either immediately after birth with respiratory distress or they may present after several hours of an asymptomatic period. The degree of respiratory distress initially may mimic any respiratory disorder. Infants may have fevers or become hypothermic.
The symptoms progressively increase in severity and may lead to shock, disseminated intravascular coagulation (DIC), and death. The CXR often resembles that of RDS, TTN, or aspiration syndromes. It is unusual to have lobar consolidation from infection in the newborn (6). Infants with an RDS-like CXR should be considered to have pneumonia until proven otherwise. The CBC and inflammatory markers have poor sensitivity. The CBC might reveal either a leukocytosis or leukopenia. A left shift with greater than 20% band forms of the total neutrophils is suggestive of infection as are neutrophilic vacuoles and toxic granulation. Elevated CRP and procalcitonin indicates a higher likelihood of infection. Platelet counts may be normal or decreased. In severe cases, a coagulopathy with elevated PT and PTT and depressed fibrinogen levels may be present. While blood culture used to be the gold standard of diagnosis, many newborns with pneumonia will have negative cultures due to maternal antibiotics. Tracheal aspirate (TA) culture may clarify the diagnosis but has been shown to have low sensitivity and specificity and therefore is not always done (11).
Treatment with an ampicillin and gentamicin is standard empiric treatment since it covers the most common organisms. Supportive care includes mechanical ventilation, supplemental oxygen, inotropic agents for hypotension and nitric oxide for infection associated pulmonary hypertension. The mortality from infections has decreased from 50% to 20% with more aggressive intensive care.
Congenital Heart Disease
Another etiology for respiratory distress in the newborn is congenital heart disease. Most infants with transposition of the great arteries, tetralogy of Fallot, and hypoplastic right or left heart syndromes, will present in the newborn period with hypoxemia. Depending on the underlying cardiac physiology, there may or may not be respiratory distress or a cardiac murmur present. The CXR might reveal cardiomegaly, an unusual cardiac silhouette, or decreased vascular markings (due to diminished pulmonary blood flow) depending on the underlying cardiac physiology. When the infant develops respiratory symptoms, it is usually from severe hypoxemia or acidosis (12). Critical congenital heart disease (CCHD) screening based on pulse oximetry screening is a simple way to screen for congenital heart disease in a newborn. The screening requires only pre and postductal oxygen saturations, respectively measured at the right hand and either foot. A saturation <95% in either measurement or a difference >3% between the two constitutes a fail or need for re-test. If preductal saturation is greater than postductal, consider persistent pulmonary hypertension and hypoplasia, coarctation, or interruption of the aortic arch. If postductal saturation is greater than preductal, consider transposition of the great vessels with coarctation of the aorta or interrupted aortic arch. The hyperoxy test may help to distinguish cyanotic heart disease with a fixed right to left shunting of deoxygenated blood into the systemic circulation from severe respiratory disease, since the former is not expected to show significant improvement with supplemental oxygen. A formal hyperoxy test may be performed by measuring right radial artery paO2 (via arterial blood gas) before and after 10 minutes of 100% inspired oxygen. An increase in paO2 by more than 150 mmHg generally excludes significant right to left shunting due to cyanotic congenital heart disease, and persistent pulmonary hypertension. An informal test may also be performed using a pulse oximeter instead of an ABG (13) but this only works if the initial room air oxygen saturation is 98% or lower. A diagnostic echocardiogram should be performed if congenital heart disease is suspected.
Therapy for cyanotic heart disease consists of medical support until definitive surgical repair can take place. Ductal dependent cases hypoplastic left heart, tricuspid atresia, transposition of the great vessels (TGV), and interrupted aortic arch, require patency of the ductus arteriosus to maintain mixing of pulmonary and systemic circulations. This is accomplished with an intravenous infusion of prostaglandin E1 infusions. Surgical shunts between the aorta and pulmonary arteries are performed to maintain mixing. Moderate oxygen supplementation to keep oxygen saturations approximately 80% or higher to maintain a fetal type circulation is attempted to preserve pulmonary function. Oxygen saturation greater than 85% can result in pulmonary vasodilation which in turn leads to pulmonary edema. Multi-staged open heart surgery is often necessary for complex cyanotic heart diseases.
Noncyanotic heart diseases such as a large ventricular septal defect (VSD) sometimes do not present at birth with respiratory distress due to the physiological increased pulmonary vascular resistance which prevents pulmonary hyperperfusion and edema. However, a murmur is often heard on the second day of life as pulmonary vascular resistance (and pressure) drops resulting in greater left to right shunting through the VSD). Clinical symptoms of respiratory distress (due to pulmonary edema) or failure to thrive may be develop weeks later if the VSD is not treated.
Pulmonary System Abnormalities
Structural abnormalities of the pulmonary system also cause respiratory distress. Pulmonary hypoplasia can result from congenital diaphragmatic hernia, preterm prolonged rupture of membranes (PPROM) at extreme prematurity, and from renal agenesis/dysgenesis with anhydramnios/oligohydramnios. Infants with congenital diaphragmatic hernia develop pulmonary hypoplasia as a result of the reduced space for lung growth and resultant maldevelopment. These infants frequently present in the immediate newborn period with respiratory distress and refractory cyanosis. The abdomen is scaphoid since the intestines are in the thorax. Bowel sounds are heard over the chest if air enters the intestines from spontaneous breathing or mask valve ventilation. The CXR reveals bowel gas pattern in the hemithorax (more commonly on the left) with a mediastinal shift to the opposite side. The heart compresses the contralateral lung which may also be hypo-inflated and hypoplastic (14). Refer to the chapter on congenital diaphragmatic hernia for more details.
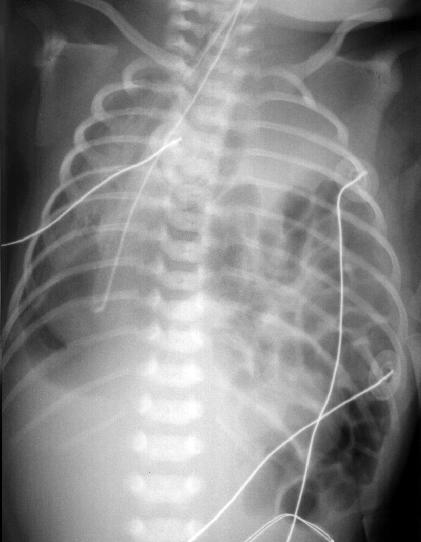
Figure 8 – Congenital diaphragmatic hernia. Bowel is visible in the left hemithorax. This compresses the lungs on both sides during fetal development predisposing the infant to pulmonary hypoplasia. Taken from Yamamoto LG. Radiology Cases In Neonatology. Copyright 1966. www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
In the case presentation at the beginning of this chapter, the gradual onset of respiratory distress which increases in severity with time is concerning for an infectious process such as pneumonia. TTN is also possible. However, given the presentation, pneumonia cannot be rulef out and empiric antibiotic therapy should be initiated.
Table 1 - Simplified summary of some of the major newborn respiratory conditions:
| RDS: Clinical factors: Prematurity, respiratory distress. CXR: Frosted glass appearance. |
| TTN: Clinical factors: Short labor, C-section delivery, tachypnea. CXR: Fluid in the fissures, central/perihilar congestion. |
| Meconium aspiration: Clinical factors: Meconium in amniotic fluid. Can lead to persistent pulmonary hypertension. CXR: Patchy Infiltrates and hyperinflation. |
| Pneumonia: Clinical factors: Prolonged rupture of membranes, maternal GBS, prematurity. CXR: Patchy Infiltrates or hazy lungs (may be identical to RDS). |
| Pneumothorax: Clinical factors: Persistent tachypnea or respiratory distress with history of positive pressure ventilation. CXR: Air between in pleural space (small to large), collapsed lung in more severe case, free air in the hemithorax. |
| Tension pneumothorax: Sudden deterioration while on positive pressure ventilation with severe hypoxia, hypotension, bradycardia. Transillumination glows on one side. Requires immediate needle decompression and tube thoracostomy prior to CXR. |
| Cyanotic congenital heart disease: Clinical factors: Heart murmur, persistent hypoxia despite supplemental oxygen. CXR: Hypo-perfused lungs (lungs appear darker), cardiomegaly or abnormal cardiac silhouette, but can also appear normal. |
| Hypoplastic lungs (pulmonary hypoplasia): Clinical factors: Respiratory distress, hypoxia, respiratory failure, pulmonary hypertension, often associated with congenital diaphragmatic hernia and/or renal anomalies. CXR: Small lungs. |
| Diaphragmatic hernia: Clinical factors: Scaphoid abdomen, respiratory distress, worsening with positive pressure ventilation. CXR: Intestines in the hemithorax (more commonly on the left side). |
Questions
1. What is the most common cause of respiratory distress in term newborns?
2. When is the onset of symptoms for transient tachypnea of the newborn and how might this help distinguish TTN from other disorders?
3. Aspiration syndromes can be caused by what types of materials?
4. The sudden onset of significant respiratory distress, hypoxia, hypotension, and bradycardia should suggest what respiratory disorder?
5. Respiratory distress syndrome of the premature infant is caused by what deficiency? What is the radiographic manifestation of this deficiency?
6. What disorder would you consider in a cyanotic infant without respiratory distress?
Related x-rays
Newborn radiographs: Available online at: www.hawaii.edu/medicine/pediatrics/neoxray/neoxray.html
References
1. Alhassen Z, Vali P, Guglani L, et al. Recent Advances in Pathophysiology and Management of Transient Tachypnea of Newborn. J Perinatol. 2021;41(1):6-16. doi: 10.1038/s41372-020-0757-3.
2. Parker TA, Kinsella JP. Chapter 47. Respiratory Disorders in the Term Infant. In: Gleason CA, Juul SE (eds). Avery’s Diseases of the Newborn. 10th ed. 2018. Elsevier, Philadelphia, PA. pp:668-677.
3. Vain NE, Batton DG. Meconium "aspiration" (or respiratory distress associated with meconium-stained amniotic fluid?). Semin Fetal Neonatal Med. 2017;22(4):214-219. doi:10.1016/j.siny.2017.04.002
4. Natarajan CK, Sankar MJ, Jain K, et al. Surfactant therapy and antibiotics in neonates with meconium aspiration syndrome: a systematic review and meta-analysis. J Perinatol. 2016;36Suppl1(Suppl 1):S49-S54. doi:10.1038/jp.2016.32
5. Committee Opinion No 689: Delivery of a Newborn With Meconium-Stained Amniotic Fluid. Obstet Gynecol. 2017;129(3):e33–e34. https://doi.org/10.1097/AOG.0000000000001950
6. Weiner GM, Zaichkin J (eds.) Textbook of Neonatal Resuscitation. 8th ed. American Academy of Pediatrics; 2021
7. Jackson JC. Chapter 46. Respiratory Disorders in the Preterm Infant. In: Gleason CA, Juul SE (eds). Avery’s Diseases of the Newborn. 10th ed. 2018. Elsevier, Philadelphia, PA. pp:653-667.
8. Whitsett JA, Kingma PS. Chapter 75. Surfactant Homeostasis: Composition and Function of Pulmonary Surfactant Lipids and Proteins. In: Polin RA, Abrman SH, Rowitch DH, et al (eds). Fetal and Neonatal Physiology. 6th ed. 2022. Elsevier, Philadelphia, PA. pp:776-787.
9. Polin RA, Carlo WA, Committee on Fetus and Newborn, American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics. 2014;133(1):156-163. doi:10.1542/peds.2013-3443
10. Bancalari E, Claure N, Jain D. Chapter 45. Neonatal Respiratory Therapy. In: Gleason CA, Juul SE (eds). Avery’s Diseases of the Newborn. 10th ed. 2018. Elsevier, Philadelphia, PA. pp:632-652.
11. Claassen CC, Keenan WJ. Challenging the "Culture" of the Tracheal Aspirate. Neoreviews. 2019;20(3):e145-e151. doi:10.1542/neo.20-3-e145
12. Scholz T, Reinking BE. Chapter 55. Congenital Heart Disease. In: Gleason CA, Juul SE (eds). Avery’s Diseases of the Newborn. 10th ed. 2018. Elsevier, Philadelphia, PA. pp:801-827.
13. Martin GR, Ewer AK, Gaviglio A, et al. Updated Strategies for Pulse Oximetry Screening for Critical Congenital Heart Disease. Pediatrics. 2020;146(1):e20191650. doi:10.1542/peds.2019-1650
14. Reed-McCullough S, Jnah AJ. Congenital Diaphragmatic Hernia: Core Review and Novel Updates. Neonatal Netw. 2021;40(5):305-312. doi:10.1891/11-T-701
Answers to questions
1. TTN
2. TTN symptoms occur soon after birth. Later onset of symptoms should suggest other disorders.
3. Meconium, blood, amniotic fluid.
4. tension pneumothorax.
5. Surfactant deficiency, which causes some alveoli to collapse next to alveoli which are emphysematous. Some atelectatic alveoli are adjacent to rigid bronchi. These conditions lead to a reticulogranular infiltrate (frosted glass) and air bronchogram pattern on the chest radiograph.
6. Cyanotic congenital heart disease.
Return to Table of Contents
University of Hawaii Department of Pediatrics Home Page