The significance of vaginal stimulation and progesterone at the time of insemination was studied in relation to the initiation of pregnancy and parturition in the golden hamster. While both are capable of supporting the initiation of pregnancy, vaginal stimuli at the time of estrus is seen crucial for the maintenance of normal pregnancy and for parturition to occur. Vaginal copulatory stimuli are seen involved in initiating mechanisms inhibiting gestational ovulation and recurrent estrus as well. Progesterone may support an apparently normal pregnancy but without vaginal stimulation normal parturition is rare. Vaginal stimulation given simultaneously with progesterone at estrus may compensate for some adverse effects of the exogenous hormone.
In 1968, Yanagimachi and I reported that pseudopregnancy in the hamster was capable of being induced by mechanical vaginal stimulation comparable to that of normal copulation. That study was followed by various investigations into those processes possibly initiated by the copulatory stimuli. One such study in which we were considering endocrine release immediately prompted by such stimuli, attempted to induce hormonally with a single injection a pseudopregnancy capable of successfully maintaining artificially inseminated ova (Diamond and Yanagimachi, 1972). Progesterone, of all the hormones tested, was found singularly successful for this use. During the course of that experiment, however, it became apparent that the pregnancy initiated with progesterone, in several regards, was not comparable to a true pregnancy. For example, the pregnancies initiated by artificially inseminating females given progesterone, despite apparently normal gestation, seldom terminated successfully; parturition was rare. Since we were previously able to initiate successful pregnancies with artificially inseminated females if they were given vaginal stimulation (Diamond and Yanagimachi, 1968), the importance of vaginal stimulation and its relation to complete pregnancy and parturition came into question. The present study investigated the comparative significance of vaginal stimulation and progesterone at the time of insemination in the initiation of pregnancy and parturition.
MATERIALS AND METHODS
Female adult golden hamsters bred in our own colony were used in this study. The females were assigned to 1 of 10 groups. These 10 groups might be considered of two main types: those in which pregnancy might be anticipated, and those in which pseudopregnancy might be expected (Table 1). Subjects in the first group were artificially inseminated only. Females of the second and third groups were artificially inseminated and given 5.0mg or 10.0 mg of aqueous progesterone subcutaneously (25mg per cc; Upjohn). Those in the fourth group received the artificial insemination after mechanical vaginal stimulation. Fifth and sixth group females were given 5or 10 mg of progesterone and artificially inseminated after vaginal stimulation. Females of the seventh group were mated normally, thus serving as controls. In the eighth group, females were mated with vasectomized males, and in the ninth group females were given only mechanical vaginal stimulation similar to copulation. Females in the tenth group were placed with males after their vaginas were sutured to prevent penetration. The next morning the sutures were removed to allow for routine inspection of vaginal discharge.
All procedures were initiated while the females were in estrus as determined by their demonstrating a strong lordosis response to an investigating male. The light schedule was adjusted to provide a 6:00 pm to 6:00 am dark cycle, and experimentation was usually conducted from 10:00 to 12:00 pm. Vaginal stimulation was accomplished as described previously using 30 stimuli (5sec each) evenly distributed with 5-sec intervals for 300 sec (Diamond and Yanagimachi, 1968). Epididymal spermatozoa for artificial insemination were obtained from normal males immediately prior to insemination and suspended in approximately 0.25-0.30 ml Tyrode’s solution. In the rat, copulation will promote sperm transport from vagina to uterus (Adler, 1969; Chester and Zucker, 1970). This has yet to be demonstrated for the hamster but the necessity for considering it pertinent here was precluded by insemination directly into both uterine horns while the females were under metaphane anesthesia. In the hamster, fertilization by direct intrauterine sperm placement is assured (Yanagimachi and Diamond, unpublished).
After the experimental procedure all females were observed daily for vaginal cyclicity, and tested at 4-day intervals for the cyclic manifestation of estrus. This was judged by pairing the test female with a male at least for 5 min and observing for the typical lordosis response. This pairing was conducted 3-4 hr into the dark phase of the cycle. The male was not allowed to do more than explore the female. In a normal estrous female this would be sufficient to elicit a strong lordosis. Pregnancy and pseudopregnancy were both evaluated by noting the inhibition of anticipated ovulation as judged by daily observation for postestrus vaginal discharge (Orsini, 1961) and testing for receptivity on the days estrus would normally reappear if the females were cycling; 4, 8, and 12 (mating = Day 0). In addition the animals were weighed daily, and their weight changes used as an index of successfully developing fetuses (Orsini, 1961). Parturition was watched for and anticipated on Day 16 after insemination (day of insemination = Day 0). Parturition in the hamster is almost invariable by day 17. For those females which had not delivered by the afternoon of Day 17, either 10 units of oxytocin (Pitocin, 10 units per cc) were administered subcutaneously in an attempt to induce parturition or they were laparotomized to examine the condition of their fetuses, and/or observed for 3 more days.
When it became apparent that parturition was seriously impaired in females which had not received vaginal stimulation, several females brought into pregnancy with progesterone alone were prepared and killed at different days of pregnancy and at Day 16 to ascertain the viability of the fetuses.
Throughout the experiment, all animals were kept in individual cages. Their quarters were air-conditioned and maintained at a temperature of 68-75F. Diet consisted of standard rat/hamster chow supplemented with dog chow and water ad libitum.
RESULTS
TABLE 1
EFFECT OF VAGINAL STIMULATION ON THE COURSE OF ENSUING PSEUDOPREGNANCY OR PREGNANCY |
|||||||
| Group | Treatment | N | Post ovulatory discharge detected Day 5, 9, or 13 |
Lordosis detected Day 4, 8, or 12 |
Vaginal resorption signs Vaginal bleeding seen |
Pregnancy weight gain (g mean, range) | Live deliveries (%) |
| (Number of females) | |||||||
| Pregnancy appropriate | |||||||
| 1 | Artificial insemination | 4 | 4 | 4 | 0 | 0 | 0 |
| 2 | 5 mg Progesterone + artificial insemination | 8 | 0 | 3 | 2 | 24 (3-38) |
25 |
| 3 | 10 mg Progesterone + artificial insemination | 8 | 0 | 1 | 1 | 42 (30-52) |
13 |
| 4 | Vaginal stimulation + artificial insemination | 9 | 0 | 1 | 0 | 36 (24-51) |
100 |
| 5 | Vaginal stimulation + 5 mg progesterone + artificial insemination | 8 | 0 | 0 | 2 | 25 (8-38) |
86 |
| 6 | Vaginal stimulation + 10 mg progesterone + artificial insemination | 7 | 0 | 0 | 2 | 40 (18-55) |
43 |
| 7 | Normal mating | 4 | 0 | 0 | 0 | 31 (26-39) |
100 |
| Pseudopregnancy appropriate | |||||||
| 8 | Mating with vasectomized males | 4 | 0a | 0a | NAb | NA | NA |
| 9 | Vaginal stimulation | 4 | 0a | 0a | NA | NA | NA |
| Other | |||||||
| 10 | Vagina sewn | 4 | 4 | 4 | NA | NA | NA |
| a Pseudopregnancy characteristically terminates by day 10. b NA = Not applicable. |
|||||||
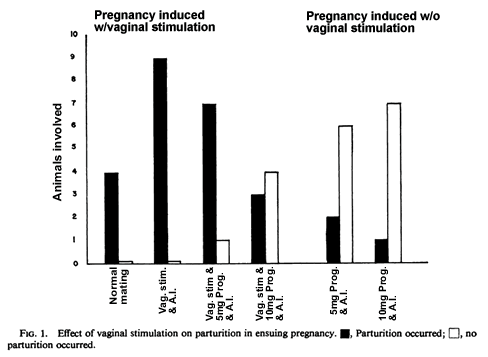
As obvious from Table 1 and Fig. 1, the only females to invariably deliver successfully were those having received only sperm and vaginal stimulation, either artificially or normally (groups 4 and 7). Parturition occurred on Day 16 in all such cases. All of the females treated with progesterone and artificially inseminated without vaginal stimulation became pregnant. Only 25% and 13% of these groups (2 and 3) delivered normally, however, despite the apparent presence of normally developing fetuses. The administration of oxytocin on Day 17 to five females of group 2 overdue in delivery was without success in bringing forth live young. Two of these five females, however, did deliver dead fetuses. Vaginal stimulation given to comparable groups at the time of progesterone and artificial insemination notably increased parturition success (groups 5 and 6); p < 0.05.
In contrast with the females receiving only sperm and vaginal stimulation where no external signs of fetal resorption were seen, 3 of the 16 females whose pregnancy was initiated with progesterone showed such signs. This fetal wastage was evidenced by weight loss first seen about Day 10 by vaginal blood originating from the uterus. It is noteworthy, however, that for all those females which received 10 mg and half of those which received 5 mg of progesterone, pregnancy weights seemed to progress normally and mothers and fetuses appeared healthy on Day 16, the expected date of delivery. By Day 17 only one female of the 10-mg group delivered; one live and seven dead babies. By Day 18 laparotomy showed that the fetuses were dead and by day 18-20 three of the still pregnant mothers died and the remaining three resorbed and were very ill.
Of those 15 females which received vaginal stimulation with progesterone (groups 5 and 6) parturition occurred after an apparently uneventful pregnancy in five. Despite apparently normal gestational weight gains, four females died after failing to deliver. Laparotomy on Day 16 of the additionally prepared pregnant females that received progesterone without vaginal stimulation invariably revealed the presence of live fetuses in utero. Some uterine signs of fetal resorption were occasionally apparent but not in amounts significantly greater than in normally pregnant females.
None of the females that had been artificially inseminated and not stimulated or given progesterone became pregnant, or even pseudopregnant (group 1). Vaginal stimulation by itself resulted in pseudo-pregnancy as anticipated (group 9). Those females with their vaginae sutured who were paired with sexually active males did not become pseudopregnant (group 10).
Judging from the appearance of a typical postovulatory vaginal discharge, ovulation was apparently inhibited on every cyclic occasion on which it might have been appropriate. The inhibition of estrus was not so universal. Estrus during pregnancy, as manifested by a typical lordosis response to an investigating male, was significantly less common (p < 0.05) in the females made pregnant by receiving normal or artificial vaginal stimulation than in the groups made pregnant with 5.0 or 10.0 mg progesterone along with the artificial insemination.
DISCUSSION
These data indicate the crucial role that vaginal stimulation plays not only in initiating mechanisms concerned with pseudopregnancy and pregnancy, but also in maintaining all aspects of pregnancy and then allowing for normal parturition. Artificial insemination without vaginal stimulation was not able to produce a successful pregnancy although fertilization occurs from the process used (Yanagimachi and Diamond, unpublished). Also pairing with a sexually active male without vaginal penetration, despite the other accompanying mechanical and psychic stimuli, does not result in pseudopregnancy changes as does vaginal stimulation alone. Confirming our earlier work (Diamond and Yanagimachi, 1972) a single dose of exogenous progesterone is capable of initiating pseudopregnancy and pregnancy mechanisms, but is not a completely adequate substitute for vaginal stimulation. Pregnancy mechanisms initiated by vaginal stimulation inhibit ovulation, recurrent estrus, and fetal resorption as well as allow for normal parturition. The progesterone initiated mechanisms of pregnancy do allow for implantation, the inhibition of ovulation, and fetal growth and maintenance to term. On the other hand, progesterone without vaginal stimulation is not comparable in allowing for the cessation of estrus and normal parturition.
Exogenously administered progesterone has long been known to support pseudopregnancy and artificially inseminated pregnancies (Diamond and Yanagimachi, 1972) and copulation has been linked to progesterone release (Avery and Stahl, 1969; Adler, Resko, and Goy, 1970). From the results here presented, however, we suggest that the initiation of pregnancy involves much more. It is postulated that the vaginal stimulation of copulation transmits neural impulses to hypothalamic circuits which directly or indirectly serve several functions. The vaginal stimuli of copulation serve to interrupt normal estrous cyclicity, stimulate maintained luteotropin release, and consequent progesterone production, and crucially, also set in motion a neural “biological clock” involved in the initiation of parturition. Since a few nonstimulated females did deliver live young, other mechanisms or circuitry than those here considered may also be involved. Since the hamster is noted for its regularity both in length of estrous cycle (Alleva et al. 1971) and gestation (Orsini, 1961), the concept of a neurological clock seems apt.
Exogenously administered progesterone is seen capable of initiating and maintaining pregnancy as well as inhibiting gestational ovulation and estrus. This role in inhibiting ovulation has been reported previously (Greenwald, 1965a, 1965b; Richards, 1966). This treatment, however, is seen very inadequate in providing for parturition. In fact, the data indicate progesterone may be detrimental. Roberts and Share (1970) have recently shown that progesterone can inhibit oxytocin release in goats. It might be considered that other exogenously administered hormonal regimens would be effective in allowing for normal parturition or that progesterone hinders normal pregnancy and, therefore, delivery. These possibilities, while real, do not negate the fact that vaginal stimulation can increase the probability that conditions are brought about which do allow for normal pregnancy and delivery. Results from groups receiving progesterone with vaginal stimulation indicate that despite possible detrimental effect of exogenous progesterone, the vaginal stimuli, nevertheless, significantly increase the chances that normal parturition will occur. With a significant difference apparent between pregnancy initiated by exogenous progesterone and one initiated by vaginal stimulation, it might be that the progesterone-induced pregnancy be termed “partial pregnancy” to differentiate it from the shorter typical “pseudopregnancy” and the more complete “true pregnancy.”
In the mouse it has recently been demonstrated that particular vaginal stimuli, the triggering by a “vaginal-code,” could initiate pseudopregnancy (Diamond, 1970). Particular stimuli also served to initiate pseudopregnancy in the hamster (Diamond and Yanagimachi, 1968). The present paper shows the role copulatory behavior plays in initiating mechanisms of true pregnancy which allow for parturition. Avery and Stahl (1969) have demonstrated that mating will induce pituitary acidophil depletion in female hamsters and this is usually associated with luteotropin release. Adler, Resko, and Goy (1970) have determined for rats that blood progesterone levels are increased after high copulatory intromission rates; it is probable that the same is true in hamsters. Harper (1970) and Grady and Greenwald (1968) have shown that the repeated administration of exogenous progesterone or gonadotropins is necessary for maintaining pregnancy in hamsters. The present data suggest that it is the stimuli of copulation that activate neural (hypothalamic) circuits capable of sustained pituitary stimulation. This stimulation would provide for proper ovarian maintenance, subsequent progesterone secretion, and eventual termination, directly or indirectly, of pregnancy. The neural impulses might work by triggering timely oxytocin release and/or effecting crucial endocrine levels via pituitary and hypothalamic feedback sensitivity.
No longer can sexual behavior and copulation be looked at simply as a means of bringing sperm to egg; rather, the behavior must be considered an indispensable and crucial component to be considered in the reproductive process and in the evaluation of reproductive failure.
Recently Vorherr (1968), Van Tienhoven (1968), and others (Turnbill et al.,1971) have reviewed many theories of how parturition might be initiated. Of the theories presented, a parturitional process related to or dependent upon vaginal stimulation was not considered. The data presented here indicate that such is the case in the hamster, perhaps involving a neural biological clock. It remains to be seen to which other species this process applies. Studies along these lines are presently under way.
ACKNOWLEDGMENTS
Appreciation for assistance in the execution of this research is particularly extended to Thomas Joseph Abrunzo; also to Jennifer Wigington and Marcia Rohter. This research was supported in part by Public Health Service Grants HD 03394, HD 02066, and HD 05179 of the National Institutes of Health, and in part by funds from the Ford Foundation. During the execution of this research the author was recipient of and received support as a Lederle Medical Faculty Fellow. Progesterone was graciously supplied by Dr. Paul W. O’Connell of the Upjohn Company, Kalamazoo, MI; oxytocin (Pitocin) was graciously supplied by Dr. A. C. Bratton, Jr. of Parke, Davis & Company Detroit, MI.
